Quote:
Originally Posted by velomonkey
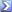
"The pressure of a gas of fixed mass and fixed volume is directly proportional to the gas's absolute temperature."
So P/T = Constant
For comparing the same substance under two different sets of conditions, the law can be written as:
P1/T1 = P2/T2
I'm also going to do this in Metric to avoid other potential errors. And I apologize that I am 20 years from doing this sort of work
12.5psi = 86.18kPa ( kilo Pascals)
75F=23.89C = 297.04K
30F=-1.11C = 272.04K
So
86.18/297.04 = P2/272.04
0.29= P2/272.04
P2=78.93kPa
78.93kPa= 11.45PSI
Temperature change from 75 to 30 degrees would result in the balls losing 1 pound of pressure per square inch.
Why don't you all complain that the Pats did a rain dance prior to the game.
|
But it was 51 degrees at game time not 30.
Len
__________________
"Evil.....is the complete lack of Empathy!"
"One of the largest obstacles to seeing truth......is wanting something too much."
|